Why does fluoride need in children's toothpaste?
Fluor is a substance that causes a lot of debates in children's toothpaste (and not only). Especially since, since the arrival of the organic trend, many brands demonize fluorine to better sell the toothpaste without fluorine. So much so that parents are very often lost before so much contradictory speeches, how can you be sure to do what it takes to protect the teeth and oral health of our children? In reality, fluoride toothpaste are not a very good idea for oral health and in particular that of our children. Why need fluorine in toothpaste? Do you need fluorine in children's toothpaste? In this article, we will answer your questions and explain our choices.
What is fluoride?
In reality, when we talk about fluorine, we generally refer to fluorine salt or the oligofluors, and not to the fluorine itself. Indeed, the pure fluorine is extremely reactive and explosive, just like its cousin chloride (belonging to the same family of elements in the periodic table, the halogens).
Halogens, but not the lamp!
The elements of this family are toxic and explosive in their isolated form. It is a French, and even Parisian chemist, Henri Moissan, who is the first to succeed in isolating fluorine and winning the Nobel Prize for Chemistry in 1906. Before him, many scientists lost their lives by trying to isolate Fluor?
Not very reassuring all that ...
Well, let's go back to children's toothpaste, or not right away, still a little story!
From 1938, there was already doubts about its beneficial effects for teeth, but in the form of salt, it became super stable. Like chloride in the form of ‘table salt’, called chloride sodium, it is very stable, it is the same for fluoride sodium often used in fluorinated toothpaste.
And yes, nobody wants toothpaste exploding in their bathroom ...
How was fluorine toothpaste born?
Well, we come back to our subject of toothpaste for children, ha yes no not immediately, still a little story! (Yes again, but you will see, it's interesting the history of fluorine)
Already in 1938, there were studies on the effects of sodium fluoride on cavities. Then it was not until 1947 that the prophylaxis of dental caries was enriched by a new fluorinated therapy.
Indeed, Frédéric Piquet adds the method of local applications of fluorinated varnishes to prevention techniques.
The first report on an effective clinical fluorine toothpaste was written in 1954 (Muhler et al).
This product combines a scan fluoride associated with an abrasive system based on calcium pyrophosphate, this toothpaste obtains global recognition of its preventive efficiency.
From then on, fluorinated toothpaste has entered our lives!
What is fluoride in toothpaste?
Its main asset concerns, as we have seen, the prevention of cavities with its topical effect (1).
Dental caries occurs when the plate, a sticky film of bacteria on the surface of the tooth, feeds with sugar and food residues to produce acid, which dissolves the surface of the tooth (it is demineralization ).
First of all, cavities is an infectious teeth disease: the danger could become very important in extreme conditions: cardiovascular concerns etc ...
For the moment, the best way to prevent them: it is to introduce topical fluorine.
The three main preventive actions of topical fluorine
- He inhibits the plate. Fluor can kill or inhibit bacteria and make them less able to produce acids drawn from carbohydrates.
- He inhibits demineralization. Fluor is integrated into crystals on the surface of the tooth, which makes this surface more resistant to acid.
- It promotes the remineralization of enamel. The process of demineralization and remineralization of enamel is constant. Fluor increases the speed of the process, and incorporating fluorine into the mineral makes it less soluble to acid.
There are many studies on the effectiveness of plant extracts and xylitol, but the results are not always very conclusive.
However, the association of sodium fluoride and xylitol in a toothpaste is more effective than fluorine alone in the remineralization of dentin (2).
What about the famous fluorosis then?
What is fluorosis?
It is an excess fluoride resulting in tasks on the teeth, it often arrives during childhood and unfortunately has no treatment. So we keep these tasks for life.
In reality, fluorosis only arrives in the event of real overdose, from overexposition to fluorine. Rest assured, the dose of consumption in cosmetics is regulated and has an extremely low risk of possible fluorosis.
Fluor, do you want it here ...
In the 1960s, scientists took a big turn by offering to give fluoride pills to children to prevent cavities.
The problem is that with this slightly extreme method, some people are undergoing a overexposure to fluoride which can induce the famous fluorosis.
Nowadays, we calmed down on fluoride stamps, but some brands offer fluoride toothpasters and use this story for marketing purposes.
And fluorine in child toothpaste?
A balance to find
Now you probably understand that the real problem of fluorine for toothpaste: it is to respect the right dose. Too little fluoride: risk of cavities, too much fluoride: risk of fluorosis ...
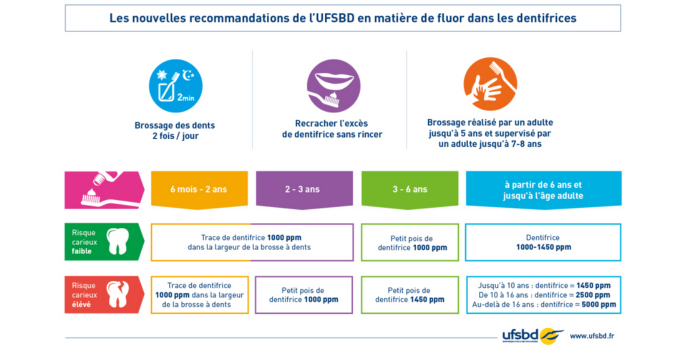
For this reason, the UFSBD regularly updates its recommendations to know the "just dose" for our children.
What the UFSBD says:
The French Union of Oral Health, the organization of the dental profession, the purpose of which is to arouse, animate and coordinate all the efforts undertaken in favor of oral health in France.
Therefore, a reference that has a lot of information on clinical cases, and the general oral health (see spreadsheet)
This is why we have followed their recommendations to formulate our toothpaste 100 % of natural origin for children.
What does PPM mean?
PPM = part per million, it is a unit of concentration to express the ultra weak dosages. It can be translated in mg/kg or percentage, 1 ppm it gives 0.0001 % (so really not much).
For example, in the food: the quantity of lead is 0.2 ppm, that is to say less than 0.00002 %.
And in child toothpaste?
For children at low risk of cavities, the UFSBD only offers 2 dosages for children from 6 months: 1000 ppm and 1450 ppm.
However, they vary the recommended amount to make the real dose (concentration x quantity) is lower.
How much toothpaste use by brushing?
Indeed, the UFSBD gives dosage recommendations, but also quantity of toothpaste to use daily according to your child's age.
In these recommendations, the quantities are measured in “trace” or “peas” to replace a precise dose as 0.25 g.
Do you see us coming?
Following your many requests for a healthy toothpaste for children, a 100 % natural toothpaste, we have created these references:
First of all, a toothpaste at 1000 ppm of fluorine for children aged 3 to 6, we are still working on a toothpaste dosed at 1450 ppm for children over 6 years old (or younger children who have had this recommendation for their dentist or doctor).
If we followed the UFSBD recommendations at the foot of the letter for these two references, we have also created a reference to 500 ppm.
Indeed, for children under 3 years of age, we have chosen to offer a less dosed toothpaste, and we will explain all this in a future article.
Fluor assessment
The benefits of fluorine on teeth health are undeniable, only in case of overdose, we can encounter various problems.
The driest means pure well enjoyed the benefits of fluorine on cavities and avoiding overdose is to make with your dentist a Fluor assessment.
In fact, for professionals, there is a fluorine daily contribution recommendation depending on age and weight.

With regard to the supply of toothpaste of our brand, we take precautions and an extreme security margin (we take the worst scenario) by considering that the toothpaste is completely ingested from the above table or the calculation has been based on an ingestion of 40 % of the toothpaste used.
How to calculate your fluorine assessment?
For the balance sheet with your dentist, you can give these figures:
For the toothpaste of 500 ppm (500 mg/kg) - size of a trace (0.125 g)
500 mg/kg x 2 times/days x 0.125 g/1000 = 0.125 mg/d
For the toothpaste of 500 ppm (500 mg/kg) - Size of a pea (0.25 g)
500 mg/kg x 2 times/days x 0.25 g/1000 = 0.30 mg/d
And for the toothpaste 1000 ppm (1000 mg/kg) - size of a pea (0.25 g)
1000 mg/kg x 2 times/days x 0.25/1000 = 0.50 mg/d
A small example:
If your 9 kg child does not yet know how to spit well, and he consumes 1 l of Vichy Saint-Yorre water (fluorine content of 8.2 mg/kg) per day, his fluorescent intake is
8.2 kg/9 kg = 0.91 mg/days/weight is a little high, because the recommended dose 0.75 mg/days/weight.
In this case, it is reasonable to reduce the dose of fluorine in toothpaste or change water to limit this contribution.

The spreadsheet is a screenshot that we found on this site which also gives you the amount of fluorine in mineral waters and in food.
If you have the least question, do not hesitate to write to us on coucou@lilikiwi.fr Or by DM on our networks, we will be happy to answer you.
Sources:
(1) Shellis RP, Duckworth RM. Studies on the Cariostatic Mechanisms of Fluoride. Int dent J. 1994; 44 (Suppl L): 263–73.
(2) Grimaud et al. Polyols in Pediatric Odontology: Interest from Xylitol. The Polyols in Pediatric Dentistry: Advantages of Xylitol. Volume 12 pediatrics archives, Issue 7, July 2005, pages 1180-1186.
(3) The effect of a stannous fluoride-containing toothpaste on caries reduction in children Joseph C. Muhler, Arthur W. Radike, William H. Nebergall and Harry G.day School of Dentistry and Department of Chemistry, Indiana University, Bloomington, Indiana, Indiana , and College of Dentistry, Ohio State University, Columbhs, Ohio